NAFDAC warns Nigerians about unregistered condom brand in circulation
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert warning Nigerians about the circulation of an unregistered condom brand known as Foula Condoms.
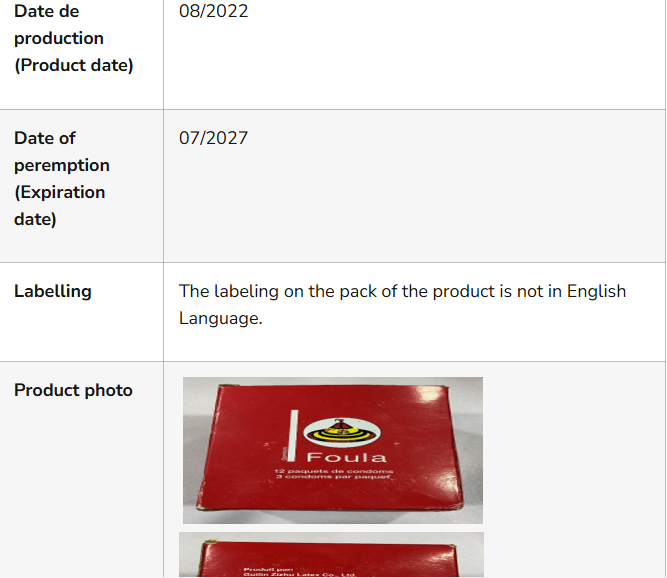
The agency on Thursday said officials from the Post-Marketing Surveillance directorate discovered Foula condoms (packaged in threes) in Abakaliki, Ebonyi State, and Zango, Katsina State.
This finding emerged during a risk-based post-marketing Surveillance study on registered condoms within the Nigerian market.
“The Foula brand of condoms is not registered by NAFDAC for use in Nigeria, and the product’s labeling is not in English, which raises additional safety concerns,” stated NAFDAC.
“Condoms are essential as an effective barrier method to prevent unintended pregnancies and protect against HIV and other sexually transmitted infections. However, they must be registered and meet quality standards to ensure safety and efficacy.” 
The agency emphasized that unregistered and poor-quality condoms can compromise public health initiatives for preventing unintended pregnancies and sexually transmitted infections.
“The illegal distribution or sale of unregistered condoms poses a risk as the safety, quality, and efficacy of the products are not guaranteed.
“The purchase and use of poor-quality condoms will adversely affect every aspect of condom promotion for the prevention of unintended pregnancy, and protection against HIV and other Sexually Transmitted Infections. If condoms leak or break, they cannot offer adequate protection,” NAFDAC added.
To counter this risk, NAFDAC has instructed all zonal directors and state coordinators to conduct further surveillance and remove unregistered products from distribution channels across the country.
NAFDAC advises Importers, distributors, retailers, healthcare professionals, and consumers are hereby advised to exercise caution and vigilance within the supply chain to avoid importing, distributing, selling, and using illegally distributed products and ensure all medical products must be obtained from authorized suppliers.
Consumers are urged to carefully verify the authenticity and condition of products before purchase.
The public is encouraged to report any suspected cases of substandard or falsified medical devices to NAFDAC at the toll-free line 0800-162-3322 or via email at [email protected]. Healthcare professionals and patients should also report any adverse effects related to medicinal products or devices through NAFDAC’s E-reporting platforms.


